| Superficial temporal artery to middle cerebral artery (STA-MCA) bypass surgery was developed as a treatment for patients with ischemic cerebrovascular disease secondary to vascular lesions that are inaccessible by carotid endarterectomy. Direct measurements of blood pressure within middle cerebral artery branches showed significantly decreased pressures in patients with intracranial occlusive disease who experienced transient ischemic attacks (TIAs) or minor stroke. A direct bypass to the middle cerebral artery would provide a collateral channel for additional blood flow to these ischemic areas and, in theory, improve perfusion.
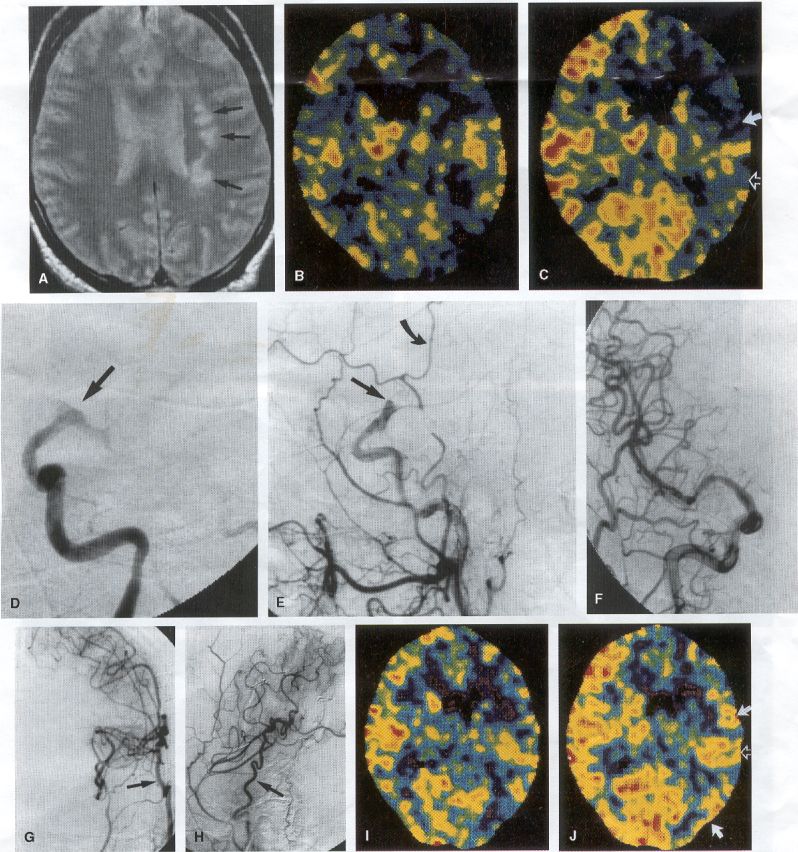
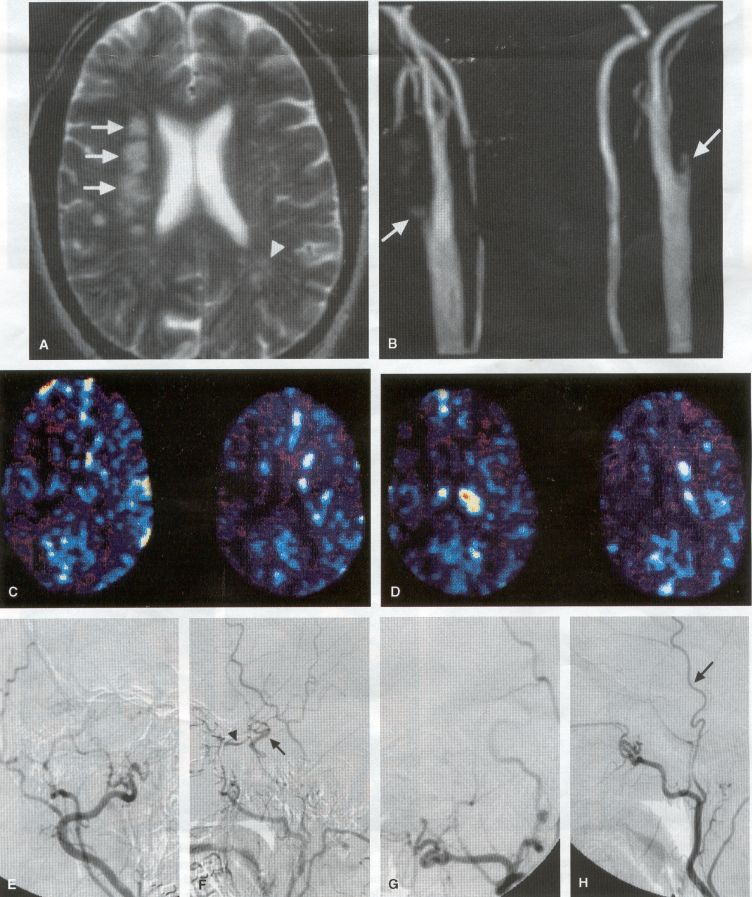
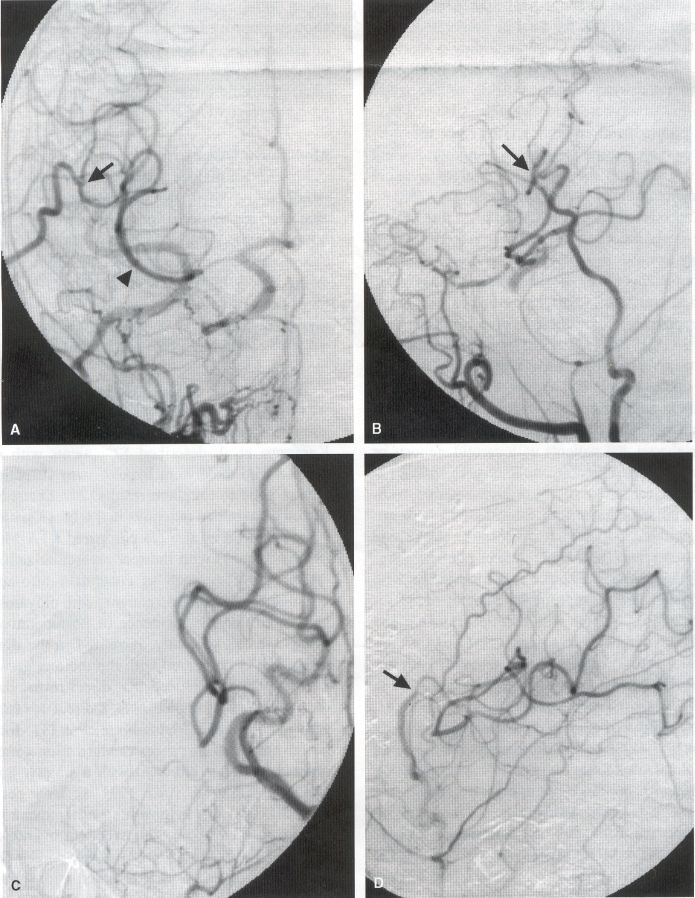
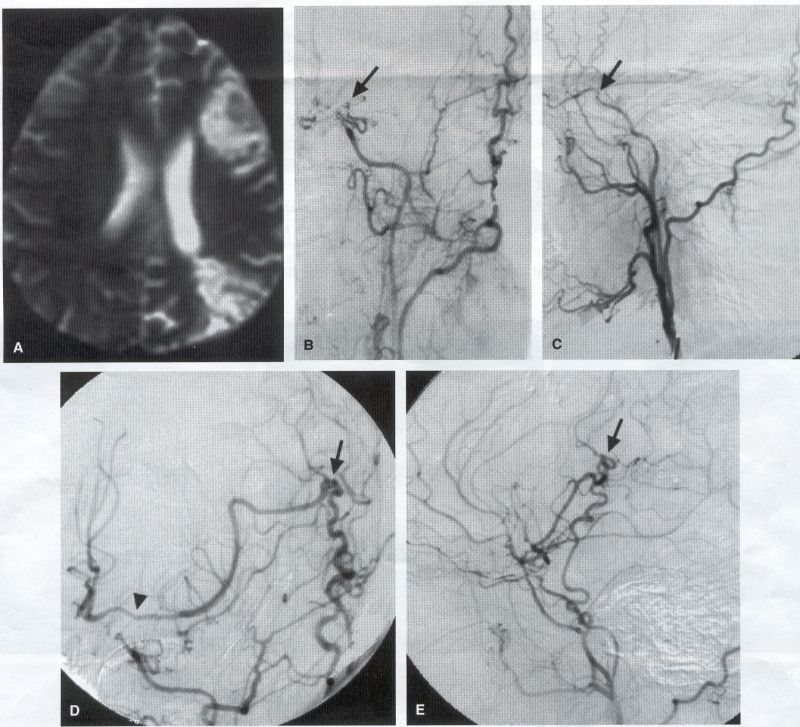
COMMON INDICATIONS FOR SUPERFICIAL TEMPORAL ARTERY TO MIDDLE CEREBRAL ARTERY BYPASS Atherosclerotic Disease The most common indication for STA-MCA bypass has been symptomatic atherosclerotic disease of the intracranial internal carotid artery (Figs. 1, 2, 3) or the MCA (Figs. 3, 4). These patients experience decreases in cerebral blood flow (CBF) that produce what has been called "misery perfusion." Ischemic regions of cortex could benefit from collateral flow and should decrease patient symptoms related to low CBF. The most frequently performed STA-MCA bypass is a direct arterial anastomosis from a branch of the STA to an MCA branch emerging from the sylvian fissure. Such grafts have been shown to result in additional blood flow of 25 to 50 mL/min, which may increase as the graft matures (9). Increased flow rates may be obtained with interposition vein grafts, which deliver flows of as much as l00 mL/min immediately after anastomosis, or by grafting the STA to more proximal M2 segments within the sylvian fissure (9,57). Yasargil et al.(67) performed the first successful microvascular STA in 1967. Subsequent studies show that the procedure had high long-term patency rates and low perioperative morbidity and mortality rates (6,7,9,11,15,44,45,58). Two of these studies documented improved regional CBF and anecdotal improvement in clinical symptoms and decreased risk for risk for future stroke (59,68). These series also revealed patency rates ranging from 87% to 100%; mortality rates of 0% to 4.4%; and low morbidity rates for strokes (2.7%), TIAs (7.3%), seizures (5.4%), hemorrhages (4%), and wound infection (1%) (6,7,9,11,15,44,45,59,68). However, these series lacked a matched control population treated with medical therapy and followed various methods of patient selection and evaluation criteria. In an attempt to clarify this issue, the International Cooperative Extracranial-lntracranial bypass study was begun in 1977 and completed in 1985 (1). This study failed to show a benefit of surgical bypass over medical management with aspirin in reducing the risk for fatal or nonfatal strokes, and it resulted in further questions regarding patient selection. The study was criticized for the large number of patients who underwent surgery outside of the study (56), the failure to separate hemodynamic causes of vascular insufficiency from embolic causes (38), the variability in obtaining routine preoperative angiograms and CBF studies (38), and the lack of a trial of medical therapy in many patients before surgical bypass (38). Recent analysis since the completion of the EC-IC cooperative study seems to show that a select group of patients may benefit from STA-MCA bypass procedures. These patients have CBF and metabolism studies, such as xenon computed tomography or positron emission tomography scans, that indicate poor or absent vascular reserve (18,19,26,33,40,47,65), and they have not responded to medical management with recurrent symptoms while taking antiplatelet agents or anticoagulation. Generally their angiograms show poor collateral flow and often an occluded ipsilateral carotid artery. Two studies have shown that patients with carotid stenosis or occlusion and impaired hemodynamic reserve have a 12-fold greater risk for stroke during a 2-year period compared with similar patients with carotid stenosis or occlusion but without impaired reserve (36% stroke rate versus 4% stroke rate) (63). Other studies confirm a significantly higher ipsilateral stroke rate for patients with carotid occlusion with compromised hemodynamic reserve (16,25,26,66). Patients with carotid occlusion and impaired hemodynamic reserve have a 6.4% to 14% annual ipsilateral stroke rate, whereas patients with severely impaired hemodynamic measurements have a 16.2% to 59.7% annual ipsilateral stroke rate (25,26,41,63,66,70). By comparison, all symptomatic patients with carotid occlusion (without CBF measurements) have an annual ipsilateral stroke rate of 1.6% to 2.8% (25,26,41,63,66,70). Usually no other alternative treatments are available to such patients with impaired reserve. With careful preoperative evaluation, they are the most likely to benefit from STA-MCA bypass. Gewirtz et al. (13) reviewed the Stanford series of 14 patients who did not respond to medical therapy after a carotid artery occlusion and who had poor hemodynamic reserve (with paradoxical steal) based on acetazolamide xenon computed tomographic scans. Thirteen of the patients had no further strokes at a mean follow-up of 49 months (range, 8-77 months). One patient (7%) experienced a stroke 2 weeks after his STA-MCA procedure when he underwent emergent coronary artery bypass surgery for unstable angina and became hypotensive during surgery. Of the nine patients who underwent a second xenon computed tomographic scan after surgery, eight patients showed improved hemodynamic reserve after acetazolamide therapy. Other investigators also found favorable clinical results after STA-MCA bypass in patients with internal carotid artery occlusion and impaired hemodynamic reserve (47.62). Moyamoya disease is a progressive disease of the internal carotid artery, MCA, and anterior cerebral artery characterized by occlusion of these vessels, which show intimal proliferation (2,17,36,64). Children with this disease usually have strokes, whereas adults have ischemic symptoms or intracranial hemorrhage. The natural history of untreated moyamoya disease is poor, with a 73% rate of major deficit or death more than 2 years after diagnosis in children (36) and a similarly poor prognosis in adults (24,43). The first STA-MCA bypass for moyamoya disease was performed by Yasargil and Yonekawa in 1972 (68), and since then several small series have been reported. Karasawa et al. described 12 patients with moyamoya disease who underwent bypass, with 10 patients (83%) having good to excellent results (24). Quest and Correll reported a similar outcome in 11 of 13 patients (85%) (43). Ishikawa et al. (22) compared STA-MCA bypass (48 procedures) with indirect revascularization methods (16 procedures), such as encephalo-duro-arterio-myosynangiosis, in pediatric patients with moyamoya disease and found that the incidence of postoperative ischemic events was significantly decreased in the direct bypass group (10%) compared with the indirect group (56%; p <0.01). They concluded that direct revascularization is the procedure of choice over indirect revascularization whenever possible. Matsushima et al. (31) found a similar advantage of direct revascularization compared with indirect methods, although with a smaller group of patients. At Stanford, since 1991 we have performed 59 (as of June 2000) STA-MCA grafts in patients with moyamoya disease (ages 5 to 63; Fig. 4) with excellent overall clinical results (5,14,55). Six patients had temporary minor worsening after operation that resolved with volume expansion, and one patient had a postoperative hemorrhage into a previously ischemic region. The mean follow-up was 43 months, ranging from 1 month to 7 years, and 95% of patients were neurologically stable or improved. Persistent TIAs developed in one patient after occlusion of a STA-MCA bypass with short vein interposition. Follow-up angiography showed that 97% of grafts were patent, and hemodynamic reserve was shown to be improved on CBF studies in most patients. There were no perioperative deaths, but one adult died of a myocardial infarction 72 months after bypass, and another adult died 18 months after an external carotid to MCA bypass using a vein interposition (from hemorrhage into ischemic brain). Of 12 children with moyamoya disease (in 19 hemispheres) treated with STA-MCA bypasses (8 after strokes and in 5 with refractory TIAs), none have experienced new strokes (mean follow-up, 35 months; range, 12-65 months) (14). Postoperative angiograms show successful revascularization of the MCA distribution in 89% of the cases, and postoperative xenon computed tomographic scans showed increased augmentation with acetazolamide compared with the results of the preoperative study (14).
PREOPERATIVE RADIOGRAPHIC EVALUATION Bilateral carotid angiograms are performed to evaluate donor and recipient vessels and collateral circulation. The size and location of optimal vessels to be used for the anastomosis are identified. A balloon test occlusion can be performed to determine the tolerance of particular vessels if planned occlusion is required. Patient who have poor results of this test need prophylactic revascularization. Xenon computed tomographic CBF studies (Figs. 1, 2, 3) or provocative induced hypotension during test occlusion may provide additional information regarding tolerance to occlusion (50,54,69). Intraoperative electroencephalography, microvascular Doppler studies, and somatosensory evoked potentials are useful adjuncts. Postoperative bypass patency is usually evaluated with conventional cerebral angiography. but recent studies show that the results of magnetic resonance angiography correlate reasonably well with those of conventional angiography (27,28,42)
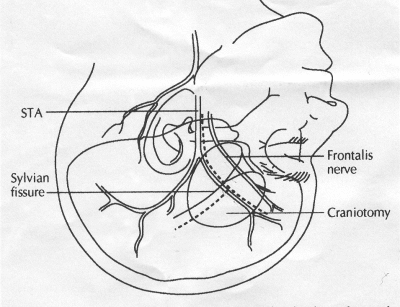
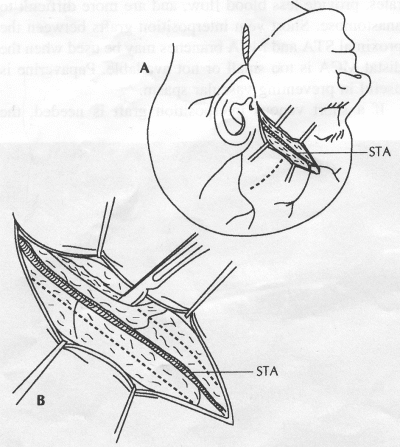
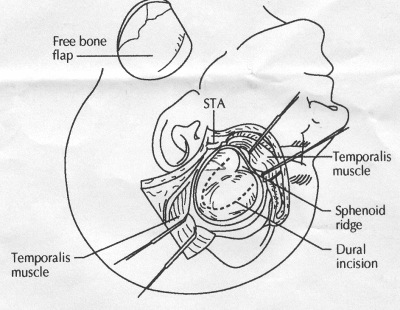
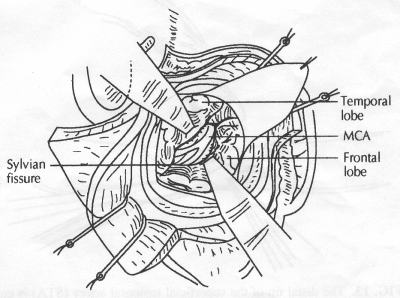
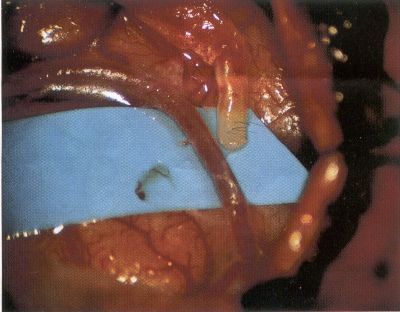
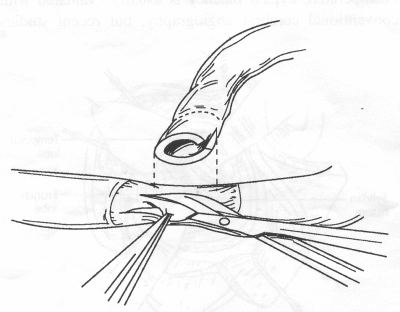
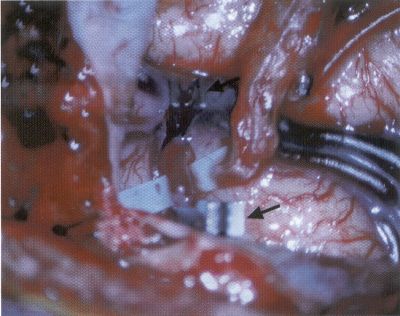
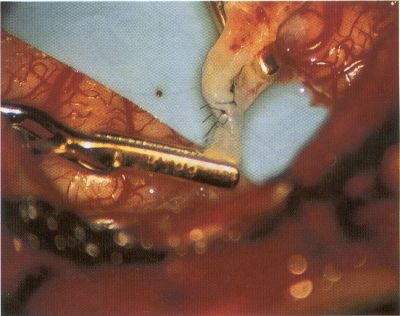
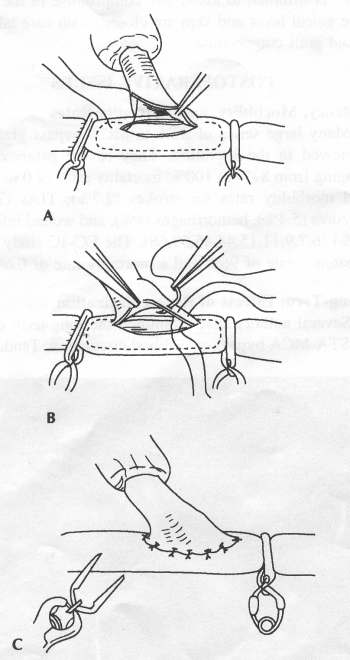
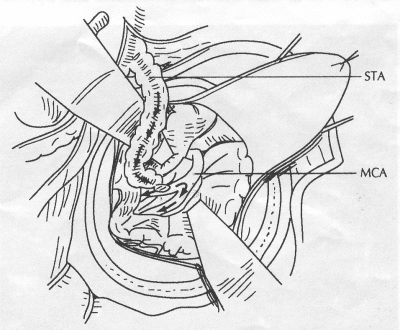
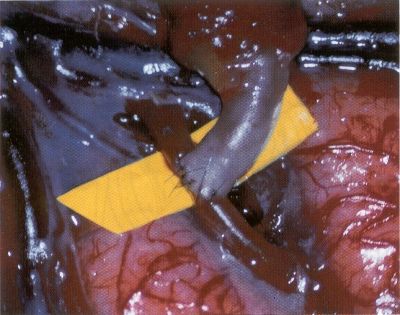
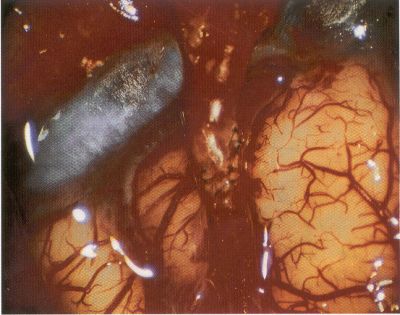
OPERATIVE TECHNIQUE General Principles Several operative generalities are applicable to STA-MCA revascularization procedures. The patient is positioned with his or her head above the heart to reduce venous cerebral congestion. Doppler ultrasound is used to identify the location of donor vessels. Hyperventilation and a-adrenergic agents are not recommended because of their vasoconstrictive effects, but mild hypothermia (33°C) and barbiturates are used routinely to provide a cerebral protective effect during arterial occlusion. Vasoconstrictive agents such as epinephrine should not be used during scalp infiltration. Intraoperative monitoring with electroencephalography and evoked potentials provide early detection of ischemic changes. Microinstruments and the operating neurosurgical microscope are used routinely for revascularization procedures. Donor vessels should be selected with a diameter ³1 mm because smaller vessels have higher occlusion rates, provide less blood flow, and are more difficult to anastomose. Short vein interposition grafts between the proximal STA and MCA branches may be used when the distal MCA is too small or not available. Papaverine is useful in preventing vascular spasm. If a short venous interposition graft is needed, the saphenous vein is the vessel of choice. It is harvested adjacent to the ankle, side branches are carefully ligated, and care is taken to avoid any laceration to the vein itself. The vein is irrigated with heparinized saline and inspected for any lacerations that would require surgical repair before use. The vein is stored in heparinized saline until it is needed. Sometimes the superficial temporal vein can be used for a short interposition graft if its diameter is sufficiently large. Hemodynamic control is the primary goal of postoperative management. Hypertension may result in excessive bleeding at the anastomotic site. Intraparenchymal hemorrhages, from increased perfusion of the brain, and a possible leak in the anastomotic site are other complications. Conversely, hypotension may cause graft occlusion, resulting in clinical ischemia. In such cases, an emergent angiogram and graft revision may be necessary. Prophylactic anticoagulants may be started on the first postoperative day, although aspirin is usually adequate. A cerebrospinal fluid leak is a potential complication, particularly when vein grafts are used, because of the deliberately loose dural closure, which prevents excessive pressure on the donor vessel. Operative Technique for Superficial Temporal Artery to Middle Cerebral Artery Bypass With or Without Short Vein Graft The patient is positioned with the head turned to the contralateral side and the temporal bone parallel to the floor, and the patient's head is fixed in a three-point Mayfield headrest. After the hair is shaved from the scalp, a Doppler ultrasound is used to mark out the course of the STA and its branches (Fig. 8). After local infiltration of the scalp, an incision is made with a #15 blade and the superficial temporal artery is identified and harvested with a generous cuff of soft tissue surrounding the artery (Fig. 9). Either the frontal or parietal STA branch may be used, depending on diameter size and suitable length. Smaller branches of the artery are isolated and carefully coagulated to prevent injury to the STA or they are tied off. The length of the artery required depends on the distance from the artery to the graft site and on whether a short vein segment will be used. A craniotomy is performed in the midfrontal-temporal bone overlying the sylvian fissure (Fig. 10). The dura is opened and recipient vessels are identified. Optimal recipient vessels have outer luminal diameters ³1 mm. A microvac subdural drain is placed to remove cerebrospinal fluid during operation. The recipient MCA/branch (usually the M3 or M4 branch emerging from the sylvian fissure) is chosen (Fig. 11) based on diameter, location (the angular MCA branch is the optimal recipient), and orientation (perpendicular to the surgeon if possible). Dissection of the arachnoid layer over a 6- to 10-mm segment exposes this vessel, and tiny branches are coagulated and divided if necessary. A high-visibility background is placed to facilitate anastomosis (Fig. 12). A temporary aneurysm clip is placed on the proximal STA donor, and the distal STA is ligated and divided at the proper length. The temporary clip is opened briefly to verify good flow through the STA. Then the STA is flushed with heparinized saline and the distal tip of this vessel is stripped of all fascial tissue and cut in an oblique manner (Fig. 13). Microvascular clips (Fig. 14) or temporary aneurysm clips (Fig. 15) are placed on each side of the recipient vessel to prevent bleeding. An arteriotomy is performed by cutting an elliptical or diamond-shaped portion of the superior wall of the recipient vessel. The recipient branch is irrigated with heparinized saline. Indigo carmine blue dye can be used to stain the edges of the MCA vessel, thereby facilitating suturing. Anastomosis is performed under the neuromicroscope using 10-0 interrupted monofilament suture (or alternatively, running 10-0 monofilament suture) (Fig. 16). Corner stitches are placed first, followed by stitches at the far wall, and finally those at the near wall. The intimal layer must be included with each interrupted stitch, but significant narrowing of the anastomotic site should be avoided. Temporary clips are removed. Significant bleeding may indicate the need for an additional stitch, whereas small amounts of bleeding may be treated with Gelfoam or Surgicel. If the donor vessel is a short vein segment, a proximal anastomosis to the STA is performed first using 10-0 or 9-0 monofilament suture. The vein must be of an appropriate length to avoid any kinks in the vein. An arteriotomy is made in the superficial temporal artery, and 10-0 monofilament sutures are used to anchor the corner stitches, followed by stitches at the back and the front walls. Once the anastomosis is complete (Figs. 17, 18, 19), a Doppler ultrasound may be used to test patency. We have found a quantitative and directional Doppler useful in the measurement of flow through the MCA segment and the STA before anastomosis, and in the STA and proximal and distal MCA segments after bypass. The dura is then closed loosely around the STA graft and the bone is trimmed to avoid any compromise of the STA. The galeal layer and skin are closed, with care taken to avoid graft compromise.
POSTOPERATIVE ISSUES Patency, Morbidity, and Mortality Rates Many large series of STA to MCA bypass grafts are reviewed in the literature. They reveal patency rates ranging from 87% to 100%, mortality rates of 0 to 4.4%, and morbidity rates for strokes (2.7% ), TIAs (7.3% ), seizures (5.4%), hemorrhages (4%), and wound infection (1%) (6,7,9,11,15,44,45,59,68). The EC-IC study noted a patency rate of 96% and a mortality rate of 0.6%. Long- Term Effects of Revascularization Several authors have examined the long-term effects of STA-MCA bypass on cerebral circulation. Tsuda et al. (60) showed (using SPECT studies) improved cortical regional CBF and cognition 3 years after the bypass. Similarly, positron emission tomography scans have shown increases in regional CBF and decreases in oxygen extraction fraction after revascularization (32,35). Complications There are several potential complications of STA-MCA bypass procedures. Ischemic changes usually result from either compression of the donor vessel by the scalp closure, graft occlusion or stenosis at the anastomosis site, or emboli. Doppler and angiography studies generally identify the cause. As discussed above, hypertension may result in anastomosis leak or parenchymal hemorrhage, and increased perfusion may cause cerebral edema and disautoregulation. In addition, because a loose dural closure is necessary to avoid any compression of the donor vessel, cerebrospinal fluid leak, pseudomeningocele, and subdural hygromas are possible, although rare. Scalp ischemia, although a theoretical risk, is extremely unusual. Finally, as with other craniotomies, systemic surgical complications include myocardial infarction, pneumonia, deep venous thrombosis, and pulmonary emboli.
CONCLUSIONS Extracranial-to-intracranial (STA-MCA) revascularization procedures have proved useful in selected patients with cerebral ischemia. Judicious preoperative evaluation coupled with attentive intraoperative techniques and postoperative management have minimized morbidity and mortality rates while improving patient outcome. Improvements in our understanding of the indications for such procedures should further refine the patient population that will benefit from these revascularization procedures.
©2003-2018
Web Vision Enterprises LLC.
All rights reserved. All information on this site is protected by international
|